A Carbon Hydrogen Bond in Ethane Is Best Described as
There is one carbon-carbon sigma bond and six carbon-hydrogen sigma bonds in C2H6. Which of the following best describes the molecules of a sample of ethane gas at room temperature.
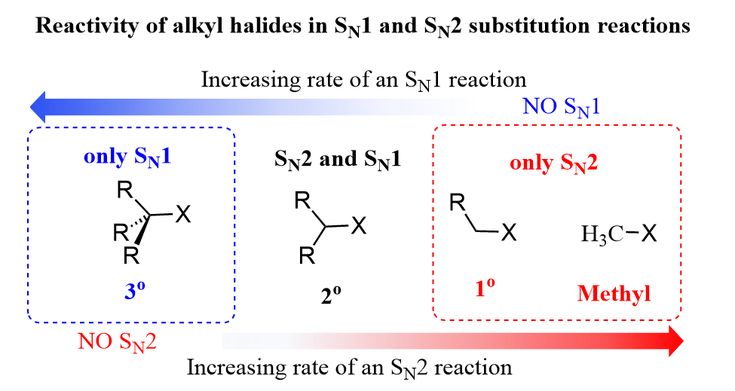
Sn1 Sn2 Reactivity Of Alkyl Halides Organic Chemistry Organic Chemistry Study Sn1 Sn2
Ethene C2H4 is an example of an unsaturated hydrocarbon with a double bond.

. In this bond which of the following best describes the charge on the carbon atom. The data above shows T1Q22 that the C-Br bond is the weakest when. Please log in or register to add a comment.
The two carbon atoms bond by merging their remaining sp 3 hybrid orbitals end-to-end to make a new molecular orbital. For Teachers for Schools for Working Scholars. Carbonhydrogen bonds have a bond length of about 109 Å and a bond energy of.
When a molecule can best be represented as a series of resonance forms each of. A Almost all of the molecules are frozen or locked in the eclipsed conformation. CH3NH2 contains a C-N bond.
A 1 B slightly positive C uncharged D slightly negative E -1. A carbon-hydrogen bond in ethane is best described as. 1 A carbon-hydrogen bond in ethane CH3CH3 is best described a A highly polar B essentially nonpolar C ionic D a multiple bond E resonance stabilized 2 When methanol CH3OH acts as a base its conjugate acid is A -CH2OH B CH30- C CH4OH D CH30H2 E CH40 3 Calculate the formal charge on nitrogen in the compound below is HHH IT H-C-CN-H H 4.
The boiling point of ethane is higher because it has stronger London dispersion forces. A carbon-hydrogen bond in ethane CH3CH3 is best described a A highly polar B essentially nonpolar C ionic D a multiple bond E resonance stabilized Question Transcribed Image Text. A carbon-hydrogen bond in ethane CH3CH3 is best described a ________.
Asked Aug 28 2020 in Chemistry by TheRussian. This completes both of their outer shells making them stable. The carbon-hydrogen bond is a bond between carbon and hydrogen atoms that can be found in many organic compounds.
Contains a C-N bond. Asked Aug 28 2020 in Chemistry by Paramedic. A carbon-hydrogen bond in ethane CH3CH3 is best described a _____.
A carbon-hydrogen bond in ethane CH3CH3 is best described a A highly polar B essentially nonpolar C ionic D a multiple bond E resonance stabilized. Methane and ethane are both made up of carbon and hydrogen. Please log in or register to answer this question.
In ethane there are 240 g of carbon for every 600 g of hydrogen a ratio of 41 by mass. A carbon-hydrogen bond in ethane CH3CH3 is best described as essentially nonpolar. What kind of molecular orbital sigma or pi results when the two atomic orbitals shown below approach.
Rank the following in order of increasing boiling pointCH3CH2NH2 CH3CH3 CH3NH2. A carbon-hydrogen bond in ethane CH3CH3 is best described as _____. A carbon-hydrogen bond in ethane CH3CH3 is best described a _____.
A multiple bond E. A highly polar B essentially nonpolar C ionic D a multiple bond E resonance stabilized. A carbon-hydrogen bond in ethane CH3CH3 is best described a _____.
What kind of orbitals result when orbitals of different atoms interact. A carbon-hydrogen bond in ethane CH3CH3 is best described as _____. A multiple bond e.
A carbon-hydrogen bond in ethane CH3CH3 is best described as essentially nonpolar The electronegativity of the elements on the periodic table increases going _____ a column and to the ____ in each row. A sample of ethane was burned completely and the water that formed had a mass of 161g. Within a given row of the periodic table electronegativity typically increases left to right across the row.
Only sigma bonds are present in ethane. When a molecule can best be. - hydrocarbons containing only single bonds - Straight chain hydrocarbons with all single bonds.
A highly polar. In this bond which of the following best describes the charge on the carbon atom. Ethane C2H6 burns with oxygen to produce carbon dioxide and water.
The molecules are rapidly interconverting between the eclipsed and staggered conformationsbut any one time slightly more of them are present in the staggered conformation. Asked Aug 28 2020 in Chemistry by Paramedic. Which of the following best describes the molecules of a sample of ethane gas at room temperature.
- An organic molecule where atoms are connected by single bonds. Ethyne acetylene C2H2 is an example of an unsaturated hydrocarbon with a triple bond. In methane there are 120 g of carbon for every 400 g of hydrogen a ratio of 31 by mass.
A carbon-hydrogen bond in ethane CH 3 CH 3 is best described as an _____ bond A highly polar B essentially nonpolar C ionic D multiple E resonance stabilized B essentially nonpolar. The bond formed by this end-to-end overlap is called a sigma bond. Asked Sep 2 2019 in Chemistry by Florence.
Which of the following best describes the molecules of a sample of ethane gas at room temperature. Option B is correct. The bonds between the carbons and hydrogens are also sigma bonds.
This bond is a covalent bond meaning that carbon shares its outer valence electrons with up to four hydrogens. The hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. The boiling point of ethane is higher because it.
A Almost all of the molecules are frozen or locked in the eclipsed conformation.

Clf3 Hybridization Chlorine Trifluoride Molecular Geometry Molecular Molecules

Hydrocarbons Class 11 Notes Chemistry Chapter 13 Learn Cbse Chemistry Chemistry Notes Chemistry Lessons

Chemical Bonding And Molecular Structure Cbse Notes For Class 11 Chemistry Learn Cbse 11thchemistrynotes Chemical Chemistry Notes Chemistry 11th Chemistry

Hydrogenation Reaction Easy Science Study Skills Functional Group Ap Chemistry

Naming Chiral Alkanes And Alkyl Halides Organic Chemistry Study Molecular Geometry Chemistry Notes

Clf3 Molecular Geometry Bond Angles Electron Geometry Molecular Geometry Molecular Molecules

C2h6 Molecular Geometry Shape And Bond Angles Ethane Molecular Geometry Molecular Geometry

No Of Carbon Name Formula Structureatoms 1 Methane Ch4 2 Ethane C2h6 3 Chemistry Basics Carbon Compounds

Sigma Pi Bonding Atomic Orbital Bonding Sigma S Pi P Bonds Sigma Pi Biochemistry Cards

Oxidation Reduction Reactions Oxidation Involves The Loss Of Electrons While Reduction Involves The Gain Of E Ensenanza De Quimica Clase De Quimica Bioquimica

Molecules Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Teaching Chemistry Chemistry Classroom Science Lessons

H2 Lewis Structure Hydrogen Molecules How To Find Out Hydrogen Atom

Alcohols 1 Nomenclature And Properties Master Organic Chemistry Organic Chemistry Chemistry Organic Chemistry Study

Hydrocarbons Simplest Form The Past Greenhouse Gases

Why Alkanes Are Called Paraffins Paraffin Data Science Chemistry

Difference Between Ethene And Ethyne Definition Properties Reactions Applications Molecular Geometry Covalent Bonding Molecular Shapes

Difference Between Ethane And Ethene Definition Properties Applications Similarities In 2022 Molecular Geometry Hydrogen Bond Molar Mass

Pin On Reactions Of Carboxylic Acids And Their Derivatives Practice Problems

Is C2h6 Polar Or Non Polar Ethane Chemical Formula Molecules Math
Comments
Post a Comment